by Guillaume BAUDOUIN
General
|
The Author: Guillaume Baudouin Following a university course between Angers, Rennes and Poitiers, Guillaume completed his thesis on the problem of termites in Paris as part of a project carried out between the IRBI (Institute of Research on the biology of the insect) and the city hall of Paris between 2013 and 2017 Interview of the author at the bottom of the page |
Termites belong to the super-order of the Dictyoptères. They form the order of the Isoptera and are related to cockroaches. Some authors equate them with the order of Blattodea. Indeed, the most recent phylogenetic analyses show that termites form a monophyletic group of Blattoptères and that their sibling group corresponds to subsocial cockroaches xylophagous (which feeds on wood) of the genus Cryptocercus (family of Cryptocercidae). Despite the strong similarity with Hymenoptera eusocial (ants, some wasps and bees), termites have a diplo-diploid breeding system and both sexes coexist within each caste. Unlike Hymenoptera that are Holometabola, termites are hétérométaboles insects or Paurométaboles.
Termites consist of Eight families (Figure 1), including the Rhinotermitidae and Termitidae that make up the most apical lineages in the phylogenetic tree, which present the most important number of species with about 300 and 2 000 Known species, respectively.
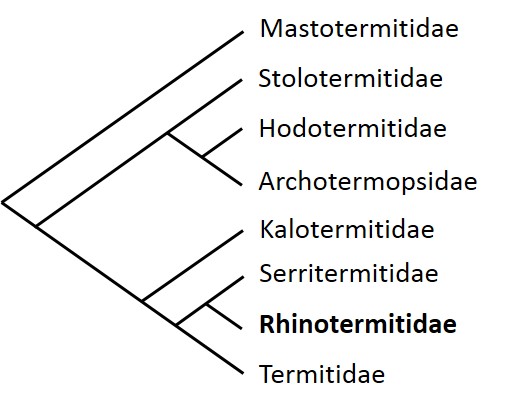
Here, we will focus mainly on termites in the Rhinotermitidae family and especially on species of the genus Reticulitermes.
Geographic Distribution & Ecology of Reticulitermes
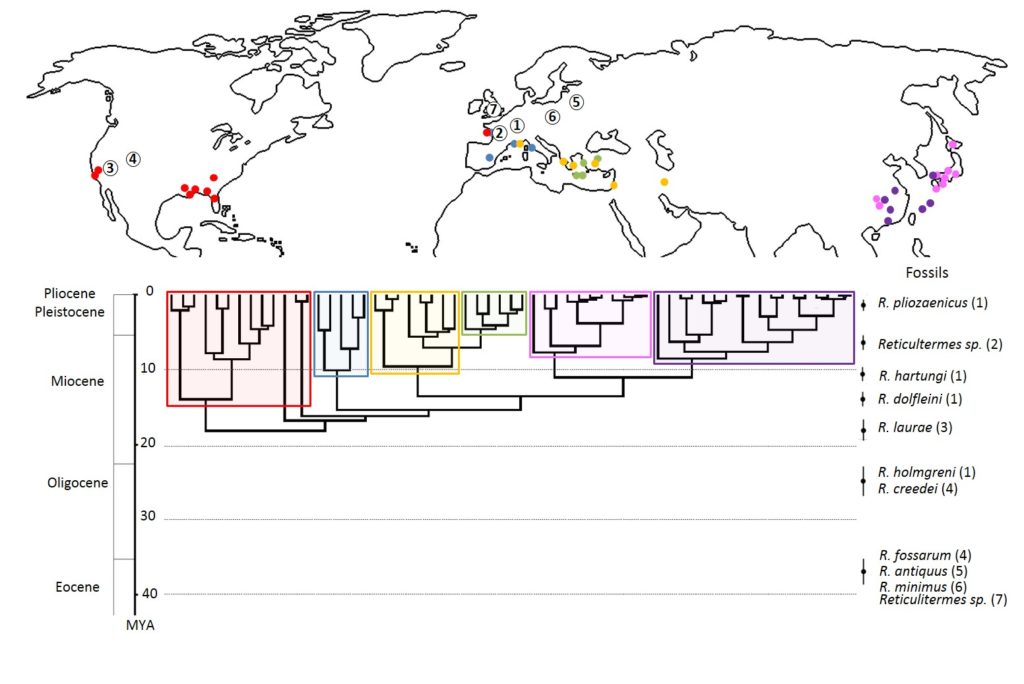
Termites of the genus Reticulitermes Holmgren (1913) belong to the family Rhinotermitidae. Diversification of this genus is estimated to be about 38 million years, where most species are believed to have originated in the North American continent. To date, there are approximately 138 species, mainly distributed in temperate regions of the world (Figure 2). Mainly located in the northern hemisphere. All species of this genus are called “underground”; They feed on dead wood and build their nests in the soil or in the wood. In nature, underground termites play an important ecological role in the decomposition of organic matter and aeration of soils.
These lucifuges termites (which flees the light) build real networks of galleries (hence their name) underground and aerial protecting them from desiccation and allowing them to reach new sources of food. During the exploration phase, only a few workers explore new territories in all directions and once the food is detected through the volatile molecules it emits, foragers undertake longer explorations and More frequent in the direction of the food source, and recruit other workers for the exploitation of this resource. However, in urban areas, Reticulitermes can be very destructive to wood-containing infrastructure.
Economic Impacts
The underground termite Group (Rhinotermitidae) is one of the termite groups with the largest number of invasive species, including species of the genera Coptotermes and Reticulitermes. On their own, the species of these two genera are responsible for 80% of the damage attributed to termites in the world. Because of their ecology (i.e., an underground life and a xylophage mode of nutrition), these species regularly infest human infrastructure. In the United States, among other things, the fight against termites and the damage they cause cost billions of dollars each year (estimated at 32 billion million in 2010).
Description of species present in Europe
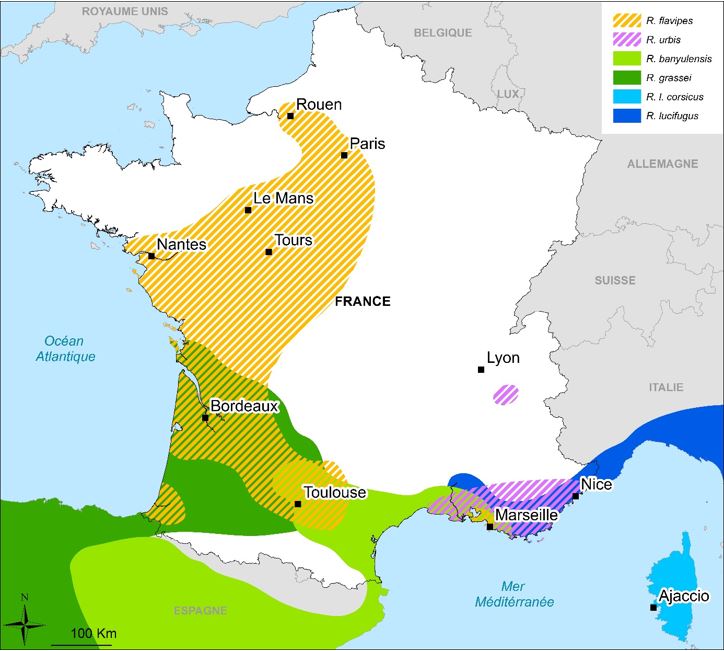
Of the six species of Reticulitermes present in France, four are native to Europe: the two subspecies R. lucifugus lucifugus and R. Lucifugus Corsicus are found on the Mediterranean periphery and, from the Iberian Peninsula, the species R. Grassei and R. Banyulensis. Also two invasive species are present in the French territory, R. Urbis and R. flavipes, respectively from the Balkans and the United States (Figure 3).
These species can be found in urban areas away from their original areas, such as R. Banyulensis in Lyon or R. Lucifugus in Bordeaux, or in Marseille where 5 of the 6 species are recovered. Recently, R. Grassei colonies were observed outside their range in the region of Tours (Project TermiCentre 2012-2015); Reticulitermes balkanensis, a Balkan species close to R. Urbis, is the only European species described so far to have not been found in French territory.
Social organization
Reticulitermes, like all termites, have an incomplete development, which makes each egg laid has a ontogenetic potential (progressive development of an organism), which can give rise to several possible developmental issues . Termite colonies are organized in caste systems, distributing individuals under different functions of colonial life. The different castes observed in termites of the genus Reticulitermes all have their particular developmental pathways (read this article). But two main lineages can be identified: The worker line, where the individuals are wingless (wingless), and the pupal line, where the individuals are brachyptères (with the development of wing buds and wings).
The working line has in principle sterile castes. It includes the caste of workers (Figures 4b) which represents the majority of individuals in the colony (about 80%). They build the nest, look for food, take care of the young and feed the other individuals in the colony. There is also the caste of soldiers, resulting from the differentiation of workers (Figure 4c). They defend the colony and represent about 2% of the colony.
The pupal line consists mainly of nymphs (figures 4d) whose successive moults can differentiate them into winged imagos, sexually mature individuals (figures 4f). These imagos, the only adults of the colony, attempt annually in the spring to seek a sexual partner and establish a new colony. These breeders are called Primary breeders: they are the ones that classically melt and ensure the breeding of the colony. There are two developmental paths that can lead to differentiation of secondary or neotenic reproducers (Figures 4e).
The neotenic are of two types: the Neotenic brachyptères (or nymphoïdes), derived from the differentiation of nymphs, and the neotenic wingless (or ergatoïdes) derived from the differentiation of workers. The latter have never been observed in the wild, but are regularly observed in laboratory-isolated colonies. These secondary breeders come to replace or complement the primary breeders and have phylopatrique reproduction.

The origin of the differentiation of the neotenic is still very poorly known. However, it is known that in the Asian species, R. speratus, the differentiation of female neotenic is inhibited by pheromones emitted by reproductive female neotenic and, conversely, these pheromones stimulate the differentiation of Male neotenic.
Very recently, this inhibition was also revealed with female ergatoïdes neotenic (derived from worker differentiation) in R. Flavipes. Furthermore, in some species of the genus, neotenic females differed from unfertilized eggs in a parthenogenetic manner.
Dispersal and foundation of new colonies
The existence of these two types of spawners (primary and secondary) is associated with two modes of possible colonial Foundation in Reticulitermes; (1) Swarming and (2) cuttings.
Swarming is the classic dispersal mode in all termite species. In the spring, the long-sheathed nymphs that made their fledge molt melanized before swarming for a nuptial flight. During the swarming, males and females go in search of a partner to found a new colony. Most often, the flight is used primarily to remove individuals from the colony from which they are derived, thus avoiding consanguineous pairing. The nuptial flight does not generally exceed a hundred meters and thus corresponds to the dispersal distance by swarming.
Once on the ground, males are looking for a female actively. This research is mainly oriented by the volatile pheromones emitted by females that allow males to be able to find them. However, pairing between individuals does not seem to take into account the interpersonal relationship and occurs randomly. Then, the partners remain in monogamous couple, copulate and go together in search of a favorable site to establish a new colony
Cuttings is a priori less common in termites, and although it has never been formally demonstrated in nature, it has been regularly suspected in many studies of Reticulitermes populations. This dispersion mode is ensured through differentiation of secondary spawners. Indeed, when colonies have abundant food resources in the vicinity of nests, the presence of neotenic allows for an increase in the exploitation of this resource. Comparing the number of offspring produced by a single queen, the presence of female neotenic will tend to increase colony productivity. However, workers and nymphs tend to look for new sources of food around their nests made up of a whole network of galleries, and some of these individuals sometimes differ in neotenic spawners, creating A network of satellite nests.
By this phenomenon, termite colonies can spread from close relatives, sometimes over several hundred meters or even in some cases up to the kilometre. In such situations, it may happen that a satellite nest is isolated from other nests and forms a new colonial entity independent of the parent colony. This mode of foundation can take place over short distances as we have just seen, but also, with the help of man, over long distances. Several studies have revealed that only a few individuals (about 50 workers or nymphs) are sufficient to build a new colony via neotenic differentiation. In an accidental manner, humans can contribute to the long-distance dispersal of these species. The cuttings has been proposed as a probable mode of dispersal in urban environments.

Dispersion system
The social organization of termite colonies can take different forms depending on the number, type (primary or secondary), sex ratio and kinship relationships among the breeders.
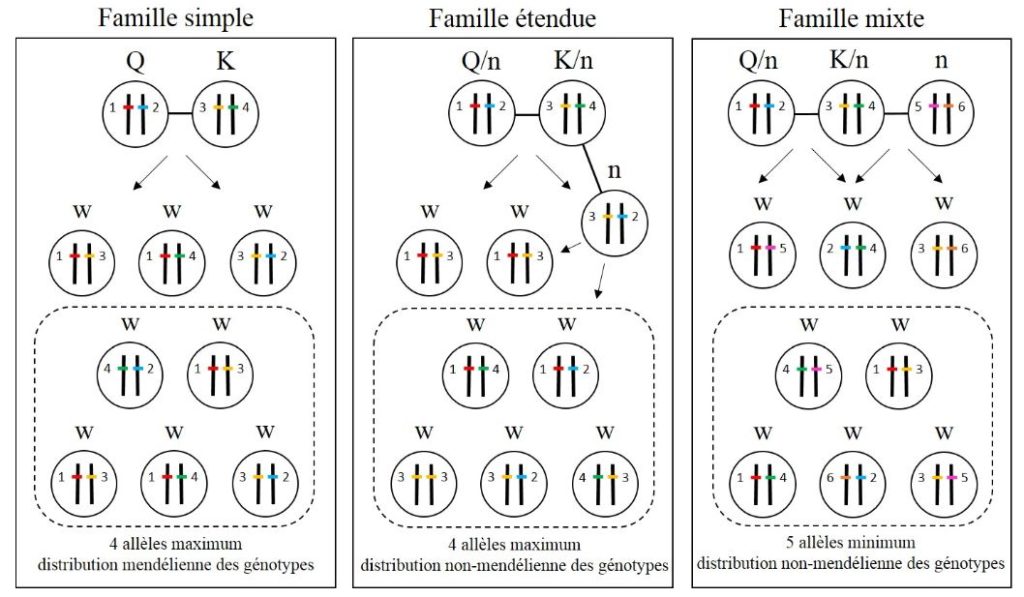
Because of their cryptic lifestyle, the spawners of underground termites, such as Reticulitermes, are generally not accessible. The use of the molecular tool allows, by collecting a dozen or twenty workers at one or more foraging sites, to identify the boundaries of the colonies and the reproductive structure of the colonies. Thanks to genetic markers such as microsatellites, it is possible to identify the number of spawners, as well as the kinship relationships of individuals within colonies by determining the distribution of genotypes and allelic frequencies in the Spawning Offspring (Figure 5). Thus, three major types of families have been defined in underground termites.
The simple family is the most commonly observed family structure in underground termites. It is composed by the monogamous primary couple, the King and Queen (usually unrelated), and their sterile offspring. Thus, the genetic analysis of a simple family never reveals more than 4 alleles at each of the loci studied. In addition, the expected genotypic frequencies in the progeny are a priori consistent with the Mendelian proportions (Figure 5).
The extended family is a family structure which, as a replacement or supplement of the two primary breeders, is the result of inbred breeding of neotenic secondary breeders. As in single families, a maximum of 4 alleles is observed at all the loci considered, representing the maximum number of alleles that can be carried by the two primary spawners at the origin of the colony. In contrast, the distribution of genotypes in the progeny of an extended family is not consistent with a Mendelian distribution (Figure 5). In fact, with the consanguineous breeding of neotenic, it is expected that the degree of kinship between descendants is greater than in a single family, and that it will increase with the number of fertile neotenic within the colony, having for Consequence a non-Mendelian distribution of genotypes in offspring. So in the colonies of this family type. In the majority of cases, however, we do not have access to spawners, and it cannot therefore be ruled out that only neotenic remain within the colony and reproduce among themselves over several overlapping inbred generations.
The mixed family is a family structure resulting from the reproduction of more than two unrelated breeders. In termites, this family structure can be obtained in several ways:
-
- By the adoption of new breeders in a mature colony
-
- By the pleometrosis, which is the foundation of a colony by more than two unrelated breeders (polygamy), usually a male and two females
- By merging two colonies. In the Reticulitermes, only the colonial fusion was observed to form a mixed family. Within these colonies, primary and/or secondary spawners from the two unrelated colonies may reproduce together. This family type can easily be identified with the use of genetic markers when at least one locus is identified with a minimum of 5 different alleles. However, the detection of these mixed families remains dependent on the rate of polymorphism of the loci used in the population studied

Although it is difficult to know, by direct observation, the nature of the breeders present in the colonies, it is possible to estimate the number of spawners within the colonies through the use of the hierarchical F-statistics. By comparing the calculated F-statistics with the studied colonies and the theoretical F-statistics obtained by simulated breeding systems, it is possible to estimate the number of spawners present in the studied colonies. The presence of neotenic in the colonies will increase individual inbreeding levels (FIC), and pairing of unrelated reproductive (e.g. mixed families) will tend to decrease the relative (r) of individuals within Colonies.
It is known in the literature that some species of the genus Reticulitermes are also capable of producing offspring by parthenogenesis thelytokous (reproduction from unfertilized female gametes (haploid) giving only Females (diploids)). The mechanism used in this asexual reproduction in Reticulitermes is Automixie with terminal fusion (fusion of 2 haploid nuclei after the second division of meiosis).
In these species, Queens exclusively use parthenogenesis to produce their neotenic daughters, although the latter breed sexually, mating with the primary male. This system of reproduction is called Asexual Queen Succession (AQS). All progeny produced by parthenogenesis in these species are neotenic females.
Individuals homozygous at all loci studied (microsatellite) are considered to be parthenogenesis products. Thus, by analyzing the rate of homozygosity in female neotenic individuals, it is possible to be able to identify species capable of parthenogenesis thelytokous. To date, the presence of AQS has been demonstrated in three species of Reticulitermes: R. Speratus, R. Virginicus, and R. Lucifugus.
Reticulitermes flavipes: History of an invasion
Geographic Distribution of R. flavipes
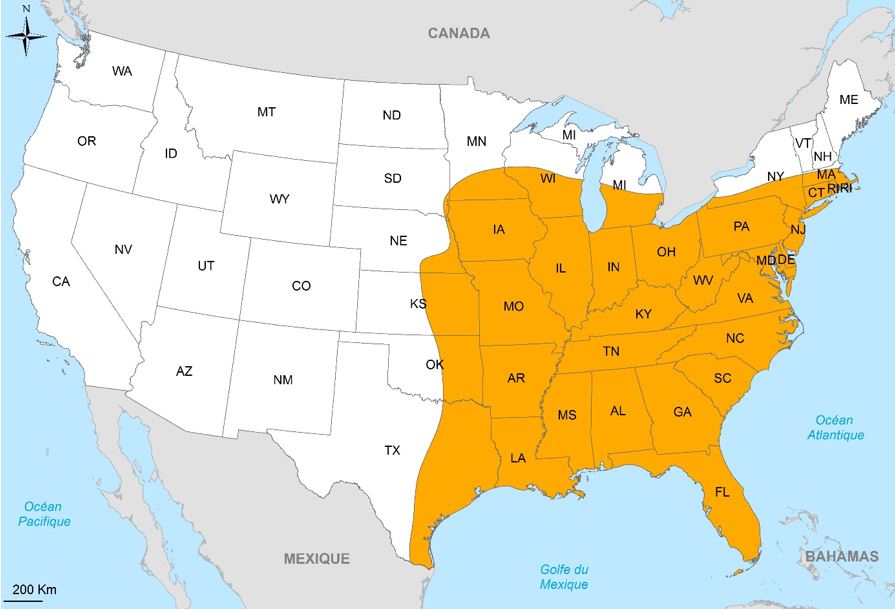
The yellow-legged termite, Reticulitermes flavipes Kollar, is a species native to the United States of America. Its range covers a large part of the eastern and southeastern United States (Figure 6). Although other species of native subterranean termites live in sympatry with this species (R. virginicus, R. Hageni, R. Hesperus, R. Maletei, R. Tibialis, and R. Nelsonae), R. Flavipes is one of the most damaging species in the United States.
This species is highly invasive and has been introduced in several parts of the world. In the United States, it has been introduced in other States than the greater east, such as California or Nebraska, as well as in other North American countries (Canada and the Bahamas) and the South (Chile and Uruguay).
In Europe, it is widespread in France (Figure 8) and has been punctually found in Germany (Hamburg), Italy (Varese) and Austria (Vienna), a country where it was first described by Kollar. In France, although this species was long regarded as European and called R. Santonensis, or Saintonge termite, several contemporary studies agreed that this “species” was actually an introduced population of R. flavipes From the United States.
An analysis of over 200 samples collected in the United States and France revealed the genetic affiliation of the French population to the American species R. flavipes. This study also revealed that all of the French infestations sampled originated in an American source population in the state of Louisiana and more specifically in the area of the city of New Orleans.
Variability of reproductive systems
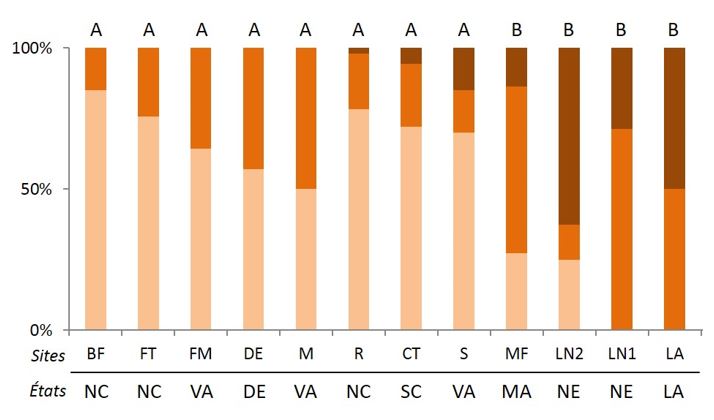
This termite has been the subject of numerous studies in its native area, particularly on reproductive systems that have been particularly well characterized. These studies have shown that there is a great variability in the proportions of the different types of families observed in this species. Although the colonies observed in its native range are mostly single families, the proportion of extended and mixed families, as well as the number of neotenic in the colonies, are variable (Figure 7).
A recent study by Vargo et al. (2013) was able to emphasize that changes in colony reproductive systems in the United States were correlated with latitudinal variations. Indeed, the emergence of extended and mixed families, and above all high inbreeding rates, as well as a greater number of spawners, would favour the persistence of colonies in higher latitudes in the north, where temperatures are more and the soil moisture more important.
In addition, seasonal variations would favour large-sized colonies during the cold period and would be limiting for single-family colonies, especially in the early stages of the colonial foundation by the adults. The large size of these colonies and their high inbreeding rates are ensured by the production of a large number of eggs from multiple female neotenic. However, the discovery in the city of New Orleans, Louisiana (Figure 7) of a very large number of extensive and mixed colonies with multiple neotenic suggests that other factors, such as the urban environment, can shape the structure Breeding in this species.
The sequel to a next article…
Interview with the author
Although termites cause many damage in the Parisian heritage, little is known about their patterns of expansion in the urban environment. It was therefore necessary to better understand their ecology in order to predict their future expansion and thus anticipate the means of prevention and struggles to be put in place. The fact that termites are social insects with a complex operating structure has really aroused interest in a population geneticist like me.
My job was to collect as many people as possible on the Parisian territory in order to identify the different populations and colonies present in the region in order to trace the kinship ties that exist between them. The objective was to identify the pathways (through the Parisian landscape) used by termites to infest new buildings and new neighbourhoods. My research has allowed to apply and adapt a new discipline: the genetics of the landscape, which combines genetics of the populations and ecology of the landscape. The use of these approaches has made it possible to identify the railway as a key element of the landscape in the dissemination of termites, both in Paris and at the scale of France.
I think that people are not informed enough about this existing urban problem since 1945 in Paris. Recently (1999) a law protects purchasers from this problem, but this is not enough to limit their spread, almost solely attributed to human activities. The species I was working on, Reticulitermes flavipes, originates in the USA where the species also causes many damage. R. Flavipes is found in its area of origin both in the forest and in the city, in France, the species is mainly present in the city. However, populations are growing in the coastal forests between the Vendée and the Charente. The ecological role of termites in the forest is major, they degrade the organic matter (wood and plants) and thus participate in the renewal, fertilization and aeration of the soils.
Not really, it is a question of informing the public so that everyone becomes aware and is an actor in this issue. Protection efforts must be mutualized: If only one of the neighbours carries out an anti-termite treatment, there is a great likelihood that other dwellings will be infested or re-infested in the following years.
Not an anecdote, but a moment that I liked very much. With my colleagues at the Paris City Hall, we had been informed of the presence of termites near the Observatoire de Paris. The observatory possessing a large ground consisting of dead wood and branches on the ground, conducive to the presence of termites, we asked to visit it. In discussing this, the person in charge told us that there are many underground gardens in the park that are not accessible to the public. Our termites being underground, so we were offered to visit the places exceptionally. Although no termites could be found, we discovered a whole network of experimentation rooms where stack clocks and a Lavoisier thermometer dating from the Revolution were stored! The insurgent thought of the Earl of Cassini was hiding nobles in his underground. |
Sources
-
- Bankhead-Dronnet S.; Olusola E.; Kutnik M.; et al (2015) Spatial structuring of the population genetics of a European subterranean termite species. Ecol Evol 5:3090 – 3102
-
- Brune A. (2014) Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol 12:168 – 180
-
- Bulmer M.S. ; Adams E.S. ; Traniello J.F.A. (2001) Variation in colony structure in the subterranean termite Reticulitermes flavipes. Behav Ecol Sociobiol 49:236 – 243
-
- Dedeine F.; Dupont S.; S. Guyot; et al (2016) historical biogeography of Reticulitermesing termites (Isoptera: Rhinotermitidae) inferred from analyses of mitochondrial and nuclear loci. Mol Phylogenet Evol 94:778 – 790
-
- Deheer C.J. ; Vargo E.L. (2004) Colony genetic organization and Colony Fusion in the termite Reticulitermes flavipes as revealed by foraging patterns over time and space. Mol Ecol 13:431 – 441
-
- Misof B.; Liu S, Meusemann K.; et al (2014) Phylogenomics solves the timing and pattern of insect evolution. Science 346:763 – 767
-
- Olusola E.; The ; Vargo E.L. ; Baldwin G.; et al (2015) Relationship between invasion success and colony breeding structure in a subterranean termite. Mol Ecol 24:2125 – 2142 (link)
-
- Vargo E.L. (2000) Polymorphism at Trinucleotide microsatellite loci in the subterranean termite Reticulitermes flavipes. Molecular-Ecology 9:817 – 829
- Vargo E.L., Leniaud L.; A. E.A. ; et al (2013) clinal variation in colony breeding structure and level of inbreeding in the subterranean termites Reticulitermes flavipes and R. Grasse. Mol Ecol 22:1447 – 1462
Author’s Articles
- Baldwin G.; Dedeine F.; Bech N.; Bankhead-Dronnet S.; Dupont S. (2017): An American termite in Paris: Temporal colony dynamics. Genetica, vol145 (6): 491-502 (link)
- Baldwin G.; Bech N.; The ; Dedeine F. (2018): Spatial and genetic distribution of a North American termite, Reticulitermes flavipes, across the landscape of Paris. Urban ecosystems, V21 (4): 751-764 (link)






